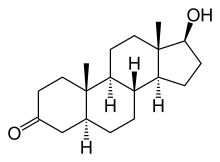
Dihydrotestosterone
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
| (5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-10 ,13-dimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17 -tetradecahydrocyclopenta[a]phenanthren-3-one | |
| Identifiers | |
| CAS number | 521-18-6 |
| ATC code | A14AA01 |
| PubChem | CID 10635 |
| ChemSpider | 10189 |
| UNII | 08J2K08A3Y |
| ChEMBL | CHEMBL422045 |
| Chemical data | |
| Formula | C19H30O2 |
| Mol. mass | 290.442 g/mol |
| SMILES | eMolecules & PubChem |
| Pharmacokinetic data | |
| Bioavailability | Oral 0-2% |
| Metabolism | Hepatic |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | X u |
| Legal status | Schedule III (US), Schedule IV (CA) |
| Routes | Intramuscular, transdermal |
| | |
Dihydrotestosterone (DHT) is an androgen, synthesized primarily in the prostate gland, testes, hair follicles, and adrenal glands by the enzyme 5α-reductase by means of reducing the 4,5 double-bond of the hormone testosterone.
Effects on sexual development
In men, approximately 5% of testosterone undergoes 5α-reduction to form the more potent androgen, dihydrotestosterone. DHT has three times greater affinity for androgen receptors than testosterone and has 15-30 times greater affinity than adrenal androgens. During embryogenesis DHT has an essential role in the formation of the male external genitalia, and in the adult DHT acts as the primary androgen in the prostate and hair follicles.
An example illustrating the significance of DHT for the development of secondary sex characteristics is the congenital 5-α-reductase (5-AR) deficiency which can result in pseudohermaphroditism. This condition results in underdeveloped male genitalia and prostate. These individuals are often raised as girls due to their lack of conspicuous male genitalia. In the onset of puberty, although their DHT levels remain very low, their testosterone levels elevate normally. Their musculature develops like that of other adults. After puberty, men with this condition have a large deficiency of pubic and body hair, and no incidence of male pattern baldness.
Unlike other androgens such as testosterone, DHT cannot be converted by the enzyme aromatase to estradiol. Therefore, it is frequently used in research settings to distinguish between the effects of testosterone caused by binding to the androgen receptor and those caused by testosterone's conversion to estradiol and subsequent binding to estrogen receptors.
Pathology
DHT is the primary contributing factor in male pattern baldness. However, female hair loss is more complex, and DHT is only one of several possible causes. Women with increased levels of DHT may develop certain androgynous male secondary sex characteristics, including a deepened voice and facial hair. DHT plays a role in the development and exacerbation of benign prostatic hyperplasia, as well as prostate cancer, by enlarging the prostate gland. Prostate growth and differentiation are highly dependent on sex steroid hormones, particularly DHT.
Treatment
The drugs belonging to the 5α-reductase inhibitors group are commonly used for the treatment of two DHT-related conditions. Dutasteride is approved for the treatment of benign prostatic hyperplasia (BPH) and is prescribed off-label for the treatment of male pattern baldness (MPB), whereas finasteride is approved for both conditions. Dutasteride is three times more potent than finasteride in inhibiting the Type II enzyme and 100 times more potent than finasteride in inhibiting the Type I form of the DHT-producing enzyme. Currently, DHT hormone replacement therapy does not exist as a treatment for DHT androgen deficiency.
Metabolism
DHT is converted to 3α-Androstanediol and 3β-Androstanediol.
See also
References
Retrieved from : http://en.wikipedia.org/wiki/Dihydrotestosterone